Solar Disinfection StudiesDrinking WaterBackground InformationThere are a few methods commonly advocated for the disinfection of drinking water at the household level. These include boiling of water for about 10 minutes, or the use of certain chlorine compounds available in the form of tablets (Halazone tablets, or calcium hypochlorite tablets) or solutions (sodium hypochlorite solutions). Water purification tablets containing tetraglycine hydroperiodide as the active ingredient (obtainable from Wisconsin Pharmacal, Milwaukee, Wisconsin 53223, USA) are also available for such use. These tablets have an expiration date, and the instructions call for the addition of 1 to 2 tablets per litre of water and waiting for 25 minutes before use.As each of these procedures has its own drawbacks, their application is extremely limited in the developing regions of the world where water-borne diseases are prevalent, and the safety of drinking water supplies cannot always be assured. Availability and costs are only part of the problem. In the case of boiling, for instance, the need for about one kilogramme of wood to boil on litre of water is totally unjustifiable in fuel-short regions already suffering from aridity and desertification. Besides, the disagreeable taste of boiled water often discourages consumers. The addition of 1 to 2 drops of 5% sodium hypochlorite solution per litre of water requires the use of a dropper and litre measure, both being uncommon devices in most homes. In view of these difficulties and constraints, it was deemed necessary to search for an alternative method for the disinfection of water on an individual basis using simple and inexpensive technology that would be more appropriate for application in the Third World.
The Experimental WorkPrompted by an understanding of the prevailing conditions and needs in the developing countries regarding the safety of water supplies in rural communities, and the rampant enteric diseases, a pertinent study was launched by us on June 4, 1979. this study, involving a series of experiments carried out over a period of more than two years, aimed at assessing the feasibility of solar disinfection of small quantities of drinking water that would satisfy the daily needs of individuals or a family. These experiments essentially consisted of subjecting artificially contaminated water in small, transparent containers, 1 to 3 litres in capacity, to direct sunlight for varying periods of exposure.A variety of containers made of transparent, clear or coloured glass or plastic, and varied in usage and shape (round, conical, and cylindrical), were used for experimental purposes. They ranged from laboratory flasks made of Pyrex glass to an assortment of ordinary bottles. Some experiments also included locally produced glass vessels with a spout commonly used for drinking water, as well as polyethylene bags (Liquid-Tite fluid containers; Falcon, Dickinson and Company, Oxnard, California, USA). The experimental water used was deliberately contaminated with municipal sewage to high levels not normally encountered even with untreated water used for drinking in rural areas. Occasionally some experimental waters were inoculated with cultured pathogenic microorganisms. In each case, the water was initially examined bacteriologically just before sunlight exposure, and at intervals of 15 to 30 minutes for a few hours during exposure of the containers to direct sunlight. All containers were kept in an upright position, except for the polyethylene bags which were laid flat on the floor, with the screw caps kept tightly in place. The other containers were left open. Removable paper labels on some of the commercial bottles were detached prior to exposure to allow penetration of light. The standard plate count and membrane filter technique were applied routinely for the estimation of total bacterial counts and coliform densities, respectively. Identical batches of water in similar containers kept in the dark, and also under room conditions of lighting, served as controls for comparison and assessment of the effect of sunlight. The experiments were generally run from 9:00 a.m. to 2:00 p.m., when the solar intensity reaches its highest levels. The roof of one of the buildings within the campus of the American University of Beirut served as the site for these experiments. The highly encouraging results of the numerous experiments demonstrated repeatedly the destructive effect of sunlight on pathogenic and non-pathogenic organisms. Some of these results and the pertinent conclusions derived from the study as a whole are highlighted hereunder for the benefit of those interested in confirming our work, and in adapting the technology to suit local conditions. The conclusions are presented somewhat in the form of constructive instructions of practical value to users of the technology, with explanations being included wherever feasible and necessary.
Results and Conclusions1. Destruction of bacteria:The results of each set of experiments have consistently confirmed the fact that the bacteria contaminating water from faecal sources are, as a general rule, susceptible to destruction upon exposure to sunlight for an adequate period of time. The rate of destruction actually depends upon a number of influencing factors. The most important ones that became clear in the course of the study include the following:
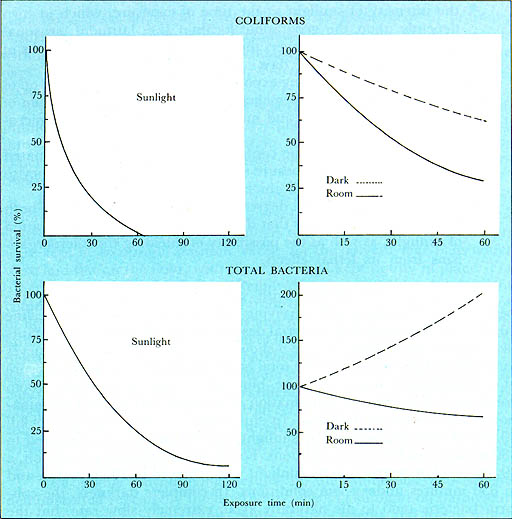
Similar patterns were obtained when a variety of other containers were used. The time required to destroy 99.9% of the coliform bacteria by exposure to sunlight ranged from 70 minutes for colourless polyethylene bags to 1050 minutes for dark brown bottles. The corresponding mean value for all types of colourless, glass or plastic containers was found to be 85 minutes. When unchlorinated batches of water inoculated with one type of enteric bacteria obtained from pure cultures were exposed to sunlight in 300 ml round Pyrex flasks, the time required for the complete destruction of each organism was found to be as follows: P. aerugenosa 15 minutes; S. flexneri 30 minutes; S. typhi and S. enteritidis 60 minutes; E. coli 75 minutes; and S. paratyphi B 90 minutes. Under similar conditions, coliform bacteria were destroyed in 80 minutes. These results indicate that, since coliform bacteria and E. coli are somewhat more resistant to the lethal effects of sunlight, they can serve as useful indicators in assessing the effect of sunlight on enteric bacteria, except for S. paratyphi B.
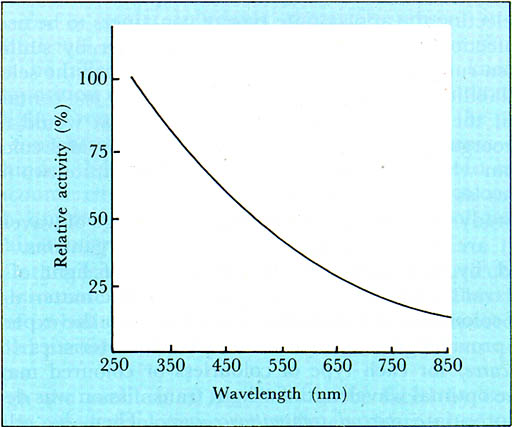
All efforts to run experiments using water inoculated with V. cholerae were unsuccessful as judged from the lack of growth in containers subjected to sunlight, room conditions, and darkness. This unfortunate outcome could be due to a number of possibilities, the foremost being, that the available pure culture itself was not viable. Because of the great importance attached to cholera, particularly in endemic areas, it would be extremely useful to repeat these trials elsewhere. Of all the pathogenic intestinal bacteria, V. cholerae are among the most sensitive to environmental stresses, and this supports the suspicion that they too are subject to the lethal effect of sunlight. As it was desirable to check on the possibility of regrowth of the inactivated bacteria, some experiments were designed to investigate this matter. The results obtained by storage for five days of disinfected water showed that inactivated coliform bacteria fail to regrow at ordinary room conditions. It is therefore assumed that already inactivated pathogenic bacteria would also fail to regrow. This would be of importance in relation to the need to store drinking water or ORS solutions without the fear of bacterial regrowth. 2. Effects on other organisms:The question often raised is whether exposure of contaminated water to sunlight in accordance with the experimental procedure adopted in our study would also lead to the destruction of microorganisms other than bacteria, e.g. enteric viruses and protozoa. It must be admitted at the outset that no straightforward answer can be offered at present in view of the fact that our study was limited to the possibility of bacterial inactivation. The lethal effect of ultraviolet light (UV) has been thoroughly investigated, and the use of UV radiation has been applied for the disinfection of water supplies in lieu of chlorination. Although information about the virucidal effect of sunlight is rather scanty, there is some evidence that viruses are inactivated by sunlight in relatively shallow ponds of water or raw sewage. The intensity of sunlight and exposure time are probably important factors. Since viruses are generally recognized to be more resistant than bacteria to the influence of disinfectants, it would be reasonable to assume that their inactivation by sunlight under our experimental conditions would require prolongation of the period of exposure. However, this matter requires further investigation. From some of our experiments using pure cultures of a variety of molds and yeasts in aqueous or brine media, it became evident that such organisms are also susceptible to sunlight. Complete destruction of Aspergillus niger, Aspergillus flavus, Candida (yeast-like fungus), and Geotrichum was achieved within three hours of exposure of suspensions to sunlight. Penicillium proved to be the most resistant as it required 6-8 hours of exposure for complete destruction. These experiments constitute part of a study aimed at the control of growth of molds and yeasts by exposure to sunlight in the process of household pickling. The inference to be drawn from these preliminary findings is the possibility of solar preservation of stock ORS solutions prepared for distribution at health care centres and dispensaries. Spore-forming organisms, not associated with disease transmission, are expected to survive the effect of sunlight until they germinate, since spores are known to be more resistant to the destructive effect of chemical disinfectants commonly used in water purification Since the thermal death point of amoebic cysts is about 50°C, contaminated water that attains a temperature of 50°C or more on exposure to sunlight would in itself ensure their destruction by this mechanism. Such temperatures are likely to be attained in regions with hot climates. 3. Impurities in water:Inorganic chemicals present in water as natural constituents, or as extraneous contaminants, are generally not expected to be affected by sunlight. Very little is known about photo-decomposition of photo-sensitive organic compounds upon exposure to sunlight. From a practical standpoint, however, the presence in reasonable concentrations of both inorganic and organic impurities would not hinder the disinfection of water by sunlight. In exceptional cases not encountered in drinking water supplies, highly coloured waters may absorb appreciable solar energy in the range of wavelengths effective against microorganisms. On the other hand, turbidity due to suspended particulate matter would hinder to some extent the penetration of sunlight. This depends on the degree of turbidity, and the depth of water being exposed. Besides, the suspended particles would protect any microorganisms adhering to their surfaces. Although the problem is not likely to be faced by communities supplied with piped drinking water, villagers deprived of such public utilities should be advised to resort to sources that yield relatively clear water. Wherever this is not feasible, and turbid surface waters from streams, ponds, or irrigation canals have to be utilized, it would be particularly important to somehow clarify the water by a convenient simple method if proper disinfection by sunlight is to be assured. Clarification not only reduces the concentration of suspended matter, but would also concurrently cause a drop in the microbial population. This can be achieved by applying traditional clarification methods often practiced by villagers in some developing countries. It is known, for instance, that in some rural areas of India the seeds of Nirmali trees (Strychnos potatorum Linn.) have been used since early times for water clarification by rubbing them against the inside walls of earthenware jars containing the water to be clarified. In fact, exposure of water to sunlight prior to filtration through charcoal for its purification is an ancient art believed to have been practiced about 2000 B.C. in India. Details about such simple indigenous household methods are presented and discussed by Samia Al Azhari Jahn in a recently published manual entitled Traditional Water Purification in Developing Countries, and published by the German Appropriate Technology Exchange, Eschborn, West Germany. Some of these methods depend upon the addition to polluted turbid water of small amounts of certain clays (known in Sudan as Rauwaq) that aid clarification. Alternative procedures include the use of a variety of native plant materials for this purpose. It remains to be pointed out that waters with relatively low microbial populations attained with or without clarification can be more rapidly and effectively decontaminated by sunlight. 4. Types of containers:There are a few simple criteria that must be applied in selecting the appropriate type of containers to be used for the proper disinfection of contaminated drinking water by sunlight. The general golden rule that needs to be followed is to base the selection not only on availability and size, but also on the need to use containers that would permit the penetration of those sun rays that would effectively destroy microorganisms. Therefore, the transparency and colour of the materials from which the containers are made constitute two important characteristics as will become clear from the text. In our study we were able to determine the range of wavelengths of sunlight that are relatively more lethal to microorganisms This was accomplished by first assessing the percentage of light of different wavelengths transmitted through the glass or plastic material of which each kind of colourless or coloured container used in the experiments is made. This provided the light transmission characteristics (or spectral transmittance curve) for each type of colourless or coloured material. In each case, the optimal wavelength for light transmission was determined from the appropriate spectral transmittance curve. Then, by relating the optimal wavelength for light transmission for each kind of container with the mean percentage of coliform organisms inactivated by exposure to sunlight under the experimental conditions, an action spectrum for coliform inactivation was obtained. This is illustrated graphically in Figure 6. From the action spectrum (Figure 6), it is obvious that the percentage of coliform bacteria destroyed decreases exponentially as the wavelength of light increases from 260 nm to 850 nm. From this it is concluded that the destruction by light of coliforms, and presumably other bacteria too, is most efficient at the lower wavelengths (260 nm to 350 nm), and is least efficient at the higher wavelengths (550 nm to 850 nm). However, we need to disregard the radiation with wavelengths below 290 nm as this component of sunlight does not reach the surface of the earth as was discussed earlier. It can be concluded, therefore, that sunlight with wavelengths ranging from 315 nm to 400 nm is the most lethal region as it accounts for about 70% of the bacterial destruction potential. This band of wavelengths is known as the near-ultraviolet region (A) of the light spectrum. Light with wavelengths falling in this region is not visible as it cannot be perceived by the eye, and is often referred to as black light. It should be noted that more of this light comes from diffuse sky light than from direct sunshine. Visible light is characterized by having wavelengths ranging from 400 nm to about 750 nm, and accounts for about 30% of the bacterial destruction capacity. It ranges in colour from violet at about 400 nm to red at about 700 nm. The sequence of colours in the series is violet, blue green, yellow, orange, and red -- a reminder of the rainbow colours.
The foregoing information is of importance in relation to the most appropriate colours of the containers to be selected that would yield optimum results in terms of microbial destruction. It is obvious that colourless plastic or glass containers are the best choice whenever available. This is because they transmit light in the near-ultraviolet region (A), which is the most lethal range, as well as in the visible range of the spectrum. Violet and blue tinted containers come next in the order of priority. Since the lethal action continues to decrease thereafter in the descending order of green > yellow > orange > red, containers with these colours should be avoided. Very light green containers may be used provided the period of exposure to sunlight is somewhat extended. Stated differently, containers made of plastic or glass with green, yellow, orange, or red colours obstruct the transmission of the most lethal rays of sunlight, unlike those that are colourless or blue. Therefore, preference should be given to containers that are either colourless or blue. Brown bottles, and to a lesser extent red ones, are recommended for the storage of actinic chemicals, i.e. those chemicals subject to chemical changes produced upon exposure to light (including sunlight) in the ultraviolet or visible spectral regions. Naturally, containers made of opaque materials such as pottery should definitely not be used at all. The wall thickness of the containers is another factor that needs to be considered. Obviously, the thicker the wall of a container the less the transmission of the effective rays of sunlight. This would in turn somewhat retard the disinfection process, thus requiring a longer solar exposure period. Glass jars, for instance, usually have thicker walls than ordinary glass bottles, especially when made in large sizes. For equal sizes, therefore, glass bottles are preferred when both are available. In practice, relatively large-sized glass jars could be used to hold several litres of water to be decontaminated by sunlight without any significant loss in the potential for disinfection provided the exposure period is somewhat prolonged. The openings of containers need not be closed or stoppered as their closure is not in any way related to the disinfection process. Nevertheless, their closure in an appropriate manner would be a desirable precaution simply to prevent the entry of such extraneous matter as dust or vermin. Experimentally it was observed that the actual shape of the containers used for solar disinfection has a slight effect on the exposure time required for proper disinfection of water or ORS solutions. Round-shaped containers have proved to be the best in that they yield slightly faster results. Other shapes (cylindrical or conical) are equally satisfactory, although their effect is slightly delayed by several minutes (a matter of no significance in practice). From the practical standpoint, round-shaped, or cylindrical containers are to be preferred to square- shaped ones for the simple reason that a rounded shape conforms better with the motion of the sun from east to west. Nevertheless, square- shaped containers can still be used satisfactorily. Containers with multi-facetted surfaces or ornamental designs that could impede the transmission of sunlight should preferably be avoided. In some cases, labels on containers may occupy such a large proportion of the exposed surface as to significantly impair the transmission of the incident rays of sunlight. Detachable labels should therefore be removed prior to sunlight exposure. Containers with large, permanent labels are to be disqualified for use; those with small labels on one side may be used provided the unlabelled surface is made to face the sun during exposure. In addition to the previously mentioned requirements pertaining to transparency, colour, shape, and size of containers, availability and cost are also important selection criteria. Wherever possible, preference should be given to locally produced containers as they are likely to be cheaper and more widely available. Used glass bottles or jars are common in most homes, even in villages. Specially designed jugs with a spout intended for drinking by pouring a stream of water into the mouth are quite popular in the Arab World at reasonable prices. These traditional jugs are made of pottery or glass. The latter come in a variety of colours, and in our experience the clear or light blue ones have proven to be useful containers for disinfection of drinking water. Their availability, low cost, and the fact that the disinfected water does not need to be transferred into another receptacle make them sufficiently attractive for the purpose intended. 6. Conditions of exposure:Indeed, it would be quite cumbersome in practice for a housewife to perform the solar disinfection operation on small batches of water a few times a day. It would be much more rational to process the quantity of drinking water estimated to be adequate for one or two days, if the necessary containers are available. For a small family of three to five members it may be feasible to run the operation on two or three occasions per week. Having secured the necessary containers, and made sure that they are of the right kind and size, they should then be properly cleaned to remove any visible dirt (and detachable labels, if labelled bottles are used). Too much dirt on the inner or outer walls of the containers would surely obstruct some of the rays of sunlight. The containers need not be washed on every occasion as long as they are kept in use and maintained in a satisfactory state of cleanliness. It is of interest to keep in mind that the inner walls of the containers with attached microbial populations will also be decontaminated by sunlight together with the water they contain. In fact, empty containers could also be decontaminated by exposure to sunlight whenever such bottles are needed for any particular purpose Incidentally, it may be of interest to note that we have also shown experimentally that dishes and similar tableware can be effectively decontaminated by exposure to sunlight for as short a time as 15 to 30 minutes. The idea is not in any way a novel one for in many parts of the Middle East, and perhaps in other regions too, it is a traditional practice for housewives to keep matresses and bed covers of sick family members for a short while in a sunny place. As a routine practice, the desired number of clean containers (e.g. bottles) are filled with water from a source normally used for domestic purposes. To ensure proper disinfection, they should then be kept in a convenient place (e.g. yard, balcony, terrace, or window) that receives direct sunlight for most of the day, or at least for the duration of the exposure. This should not present any problem in rural areas where open spaces are amply available. The containers should be properly spaced to avoid shadows. Because the intensity of sunlight is greatest between ten o'clock in the morning and two o'clock in the afternoon, it would be wise to use that period for exposure of the containers. Since it is not practical for a housewife in a rural setting to keep time properly, she could expose the containers as early in the morning as desired, and remove them in the afternoon. Alternatively, she could remove the required number of containers in the afternoon for use, while the rest are kept until needed the next day. Adoption of such regimes would certainly not lead to any undesirable outcome because of over-exposure. They are merely aimed at simplifying matters. For maximum benefit from the disinfection action of sunlight, the containers should be kept in a slanted manner with their greatest surfaces (if not round or cylindrical) made to face the sun rather than keeping them in an upright or flat position. A special rack designed to hold the containers (e.g. bottles) in a slanted fashion would then be necessary, the optimal angle of inclination from the horizontal being equal to the latitude. Such a stringent requirement is neither practical nor essential, and the benefit derived therefrom is not justifiable. The advantage to be gained can be compensated by simply prolonging the exposure period. Regions having some 300 or so sunny days with clear skies per year are naturally best suited for the optimal utilization of solar energy for the disinfection of drinking water, as well as other applications. It is there that cloud formation would present no serious problems throughout the greater part of the year. Obviously, clouds tend to reduce the intensity of direct sunlight to some extent, the magnitude of the reduction being dependent on cloud coverage. Under such conditions, however, the scattered rays of sunlight producing diffuse daylight would still exhibit germicidal action, but at a slower rate. We have, in fact, repeatedly demonstrated experimentally that the germicidal action does take place even in indoor areas with reasonable natural light. The germicidal action, however, is roughly about ten times slower than that occuring in direct sunlight. During cloudy days, therefore, all that is necessary would be to prolong the exposure period from a minimum of one hour to several hours. The routine procedure indicated above involving exposure from morning to afternoon would be more than adequate to account for this requirement, even on days with reasonable cloud coverage. In regions with warm climates and high solar intensities, the water undergoing decontamination could become unpleasantly warm for drinking by the end of the exposure period. The rise in temperature is actually caused by the red and infrared rays of light. Bluish containers would tend to cut off most of this kind of solar energy and, thus, minimize the increase in temperature. However, the issue is somewhat different with containers made of transparent, clear glass or plastic, because such materials do allow the passage of the heat-producing energy. In our experience m Beirut, the temperature of small portions of water in Pyrex glass containers rose by about 5°C from 25°C to 30°C. In hotter places the temperature may rise to 50°C or 60°C. These pasteurization temperatures, if attained, would be an additional asset in helping to destroy such organisms as amoebic protozoa. In the event that the water becomes unpleasantly warm for drinking, it would be necessary to keep it in a shady cool place before use. Alternatively, if the exposed containers are routinely kept overnight, the water could then be ready for use in the morning. The problem then arises only in case of emergencies. But, in the case of ORS solutions, warmth may be an advantage in palatability.
|