Halosol Water DisinfectionTreating water with large doses of sodium hypochlorite or iodine solutions and subsequent exposure to solar radiation, aptly designated as "halosol" technique, was developed at the American University in Beirut (1979-1982). It was intended to be an efficacious disinfection method for small volumes of heavily polluted water with the concomitant removal of excess halogen by solar irradiation. This would also help to avoid taste and odour complaints.
Batch systemDuring the batch experiments, up to 5 L of halogenated water containing chlorine or iodine residuals of <=7 mg/L were exposed to sunlight in transparent containers made of colourless or blue-tinted glass or plastic, showing efficient halogen removal. For instance, the T50 and T99 values for dechlorination were 11 and 72 min (32 and 215 min for deiodination), respectively. In contrast, the decay reaction occurring under normal room illumination was slower, and complete darkness (or the use of dark brown containers) retarded it. The percentage mean values for the chlorine residuals decomposed photochemically, when regressed exponentially against the specific wavelengths of light that yield at least 50% transmittance, revealed that
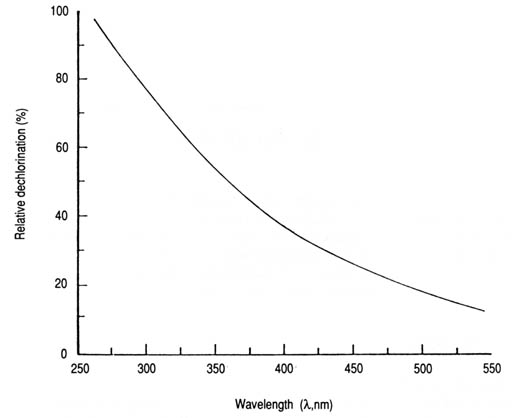 Fig. 28. Relative dechlorination (%) of water exposed to solar radiation in coloured glass containers as a function of wavelength. Relative dechlorination (%) = 612e-0.007[lambda] (n = 5, r = -0.914, p < 0.05). An inverse linear relationship between total chlorine residual (TCR, percent) and chlorinated water temperature (T) in the range of 20-70°C was observed. A mean value of 4.7% drop in the initial TCR for each 1O°C rise in the temperature above 207deg;C was obtained. The relevant linear expression is as follows:
Because the rise in temperature of water exposed to sunlight did not generally exceed 10°C in experiments run up to 180 min, the major photodechlorination effect was considered to be due to solar radiation. The relative rates for raising chlorinated water temperature in containers shielded with coloured plastic cylinders and exposed to sunlight were as follows: colourless (100%) > red (83%) > green (71%) > yellow (69%) > orange (62%) > blue-green (58%) > blue (46%). As TCR diminished with length of exposure, initial pH also decreased. This is presumed to be induced by the phototransformation of the weak HOCl to the strong HCl, liberating oxygen (Driver 1960):
2HOCl + sunlight --> 2HCl + O2 The linear expression derived from the experimental data for this effect was as follows:
It was found that an intervening glass reduced the photodechlorination process, which was 2.5-3 times more efficient for containers placed in front of a closed glass window than for those placed behind. For instance, T99 values for percentage chlorine reduction were 80 and 230 min for containers placed in front and behind the window, respectively. Photodechlorination in round 3-L flasks was found to be 10% more efficient (T99 = 117 min) than for conical flasks (T99 = 130 min). This difference may be due to the more favourable angles of incidence of solar radiation on spherical containers, which intercept light equally from all directions. From the batch experiment results, it was concluded that some of the important advantages of the halosol technique are the following:
Dechlorination kineticsThe reactions of halogens with water as a function of pH and temperature could be complicated by the formation of a variety of species. The formation of chlorine monoxide (Cl20), for instance, as a very reactive species has been postulated to occur at a pH below 8 (Reinhard and Stumm 1980):
where C0 is initial halogen residual concentration (milligrams per litre), C is halogen residual concentration (milligrams per litre) after exposure, C/C0 is the ratio of halogen residuals, K is the photodecomposition rate constant (square centimetres per microwatt minute, cm2/µW min), I is the incident solar UV-A intensity (microwatts per square centimetre), T is exposure time or photoreaction time (minutes), and e is equal to 2.7182. As for equations 3 and S, this expression may be transformed:
where R is the halogen residual remaining (percent) and F is fluence (watt hours per square metre or microwatt minutes per square centimetre). Other symbols and units are as previously defined for equation 9. The exponential decay curves representing the decrease in chlorine concentration as a function of solar UV-A radiation intensity or fluence were based on the mean values obtained for a group of experiments run under varied conditions of sunlight and exposure time. The computed values were derived through either equations 10 or 11.
MethodologyType I solar reactors (Fig. 15) were used independently to test the photodehalogenation process in continuous-flow systems. Commercial sodium hypochlorite solution (Clorox, Clorox Co., Casa Mitjana, Spain) was added gradually to the 150 L of tap water in the storage reservoir. A contact time of 30 min or more was allowed for the completion of any oxidative reactions. The same test water was used, but some differences in quality were noted (Table 5).
The total active chlorine concentration was determined in each case by amperometric titration (APHA 1985) using a CL Titrimeter 397 (Fisher Scientific Co., Pittsburgh, PA, USA). The solar UV-A intensity measurements, the experimental procedure, and data analysis were similar to those previously described. The measured parameters (see sample data sheet in Appendix 15) included local time, flow rate (litres per hour), solar UV-A intensity (watts per square metre), and TCR (milligrams per litre). Temperature was not recorded. Exposure time or residence time, fluence, and percentage chlorine remaining were calculated.
ResultsThe experiment with the batch halosol system prepared the ground for the continuous-flow system. A total of 11 experiments was run on solar reactors IA and IB (21 April-1 July 1986). The closely related results of these experiments justified pooling the experimental data for statistical analysis and evaluation.The photochemical decay rates and other relevant values were computed from equations 10 and 11 using mean values to minimize any bias introduced inadvertently. These equations are considered mathematically good approximations for the assumed uniform flow (plug flow) of the chlorinated water through the reactors. The solar exposure time (T) calculated for each interval was also based on uniform, laminar flow. The obtained data (Appendices 16 and 17) provided sufficient variability for reliable and statistically significant results (p < 0.001). The photolysis curve of HOCl (Fig. 29), and possibly other species such as chlorine monoxide, represents the percentage of the remaining chlorine residual as a function of fluence. A mean photodechlorination rate (k) of 0.238 m2/W h was calculated for initial chlorine concentrations ranging from 1.63 to 7.65 mg/L. The calculated fluences required to effect 90 and 99% reductions in the initial chlorine residual were found to be 9.65 and 19.30 W h/m2, respectively.
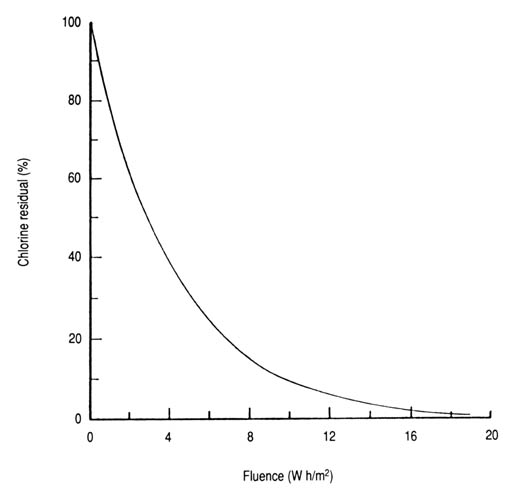 Fig. 29 Dechlorination (% of remaining chlorine residuals) as a function of solar UV-A fluence. Based on data from Appendix 16 and equation 9: R = 100e-0.238IT (n = 12, r = -0.985, p < 0.001).
DiscussionThe fundamental concept of the halosol system for water disinfection is based on the use of a combined disinfection mode through the use of two or more cooperative biocidal agents: halogens (particularly chlorine) and solar radiation. This system enhances the biocidal action of the individual agents and widens their scope of effectiveness when combined.Waterborne pathogenic bacteria are more efficiently destroyed by halogens (chlorine, iodine, and some of their derivatives) than are viruses, spores, ova, and protozoa. This shortcoming may lead to partial success when these disinfectants are applied to highly polluted waters in endemic areas. Sunlight has proven to have similar bactericidal properties (Chamberlin and Mitchell 1978; Mitchell and Chamberlin 1978; Harm 1980; Acra et al. 1980, 1984), but its action on viruses, ova, protozoa, and spores remains unclear. Nevertheless, combined effects of the two forms of disinfection (halosol process) could destroy highly resistant microorganisms and their latent stages. This hypothesis is not unfounded in view of the known germicidal effects of solar radiation and free chlorine residuals, and the possible involvement of highly reactive photochemical by-products such as singlet oxygen and chlorine monoxide. Whether or not a synergistic interaction takes place among these biocidal agents remains to be investigated. During the haloform reaction, expected to occur between the natural bromides and the active chlorine residual in chlorinated water, the liberated hypobromite (HOBr) will help the harmful haloforms form. This has to be considered carefully. Solar radiation could inhibit this process by enhancing the photodecomposition of hypobromite. In addition, photosensitive organic pollutants, humates, and other trihalomethane precursors present in water could be equally decomposed by photolysis. Removing excess halogen residuals helps in alleviating consumer complaints. Photodechlorination could also be useful for chlorinated waters used in food-processing plants or for dechlorinating effluent before discharge. Different physical and chemical water characteristics could affect the halosol process by influencing the performance of each disinfectant. Although the water used for the halosol experiments was taken from the same source as in other experiments, some of its characteristics had changed (Tables 2 and 5) as the fresh water supply is supplemented with saline groundwater during the dry season (Acra et al. 1985). Temperature and pH influence reactions of halogens in water and the formation of active halogen residuals. These, in turn, would affect the inactivation of the waterborne organisms (Sconce 1962; Jolley et al. 1980; Cheremissinoff et al. 1981). Acidic waters and high temperatures are detrimental to many microorganisms and tend to enhance their inactivation by disinfectants (Berg 1978; Mitchell 1978; Gaudy and Gaudy 1980). The more active form of chlorine (HOCl) predominates at pH below 7.53, whereas the weaker hypochlorite ion (OCl- is the predominant species above this pH (Reinhard and Stumm 1980). Because the rise of temperature in the test water in the reactor could not have exceeded 10°C, the potential effect on the chlorine-water reaction and on the germicidal action of the active chlorine residual might be enhanced to some extent. A mean turbidity level of 1.04 NTU is not sufficiently high to markedly retard transmission of sunlight through water in the solar reactor. In any case, suspended mineral sediments in water layers of <=25 cm can still induce photolysis by scattered solar radiation (Miller and Zepp 1979). Salts, heavy metals, and hardness of water could stress or inhibit bacteria and viruses; this issue remains partially unresolved. It appears that different microorganisms respond differently to variations in these factors (Iverson and Brinckman 1978; Katzenelson 1978; Kenner 1978; Mitchell and Chamberlin 1978; Le Chevallier and McFeters 1985). It is not evident, however, that the high hardness level of the experimental water could have affected the reported halosol results. When carried out with small batches, the halosol process revealed the following:
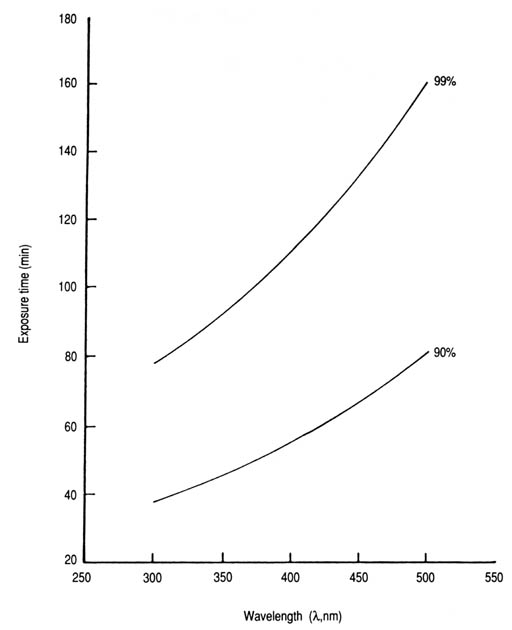 Fig. 30. Time required to remove 90, and 99% of the chlorine residual in water exposed to solar radiation in transparent glass containers as a function of wavelength. T90 = 12.5e0.0041[lambda] (n = 8, r = 0.785, p < 0.05); T99 = 26.0e0.0041[lambda] (n = 8, r = 0.783, p < 0.05). As solar intensity increases, exposure time would have to be decreased proportionally to keep the product, or fluence, constant. In the flow-through system, this would have to be handled by regulating the rate of flow. The calculated T values based on the experimental data for different solar UV-A intensities and percentages of remaining chlorine residuals (Appendix 18) will be useful in this regard. However, there could be difficulties in practice because the percentage reduction in chlorine residuals tends to vary with the time of day and, hence, with the prevailing solar intensity or fluence (Fig. 31). Suggestions for resolving this issue for the solar disinfection of drinking water using solar reactors are also applicable. It is expected that other halogens, particularly iodine and bromine, would display a pattern of response to solar radiation similar to that of chlorine.
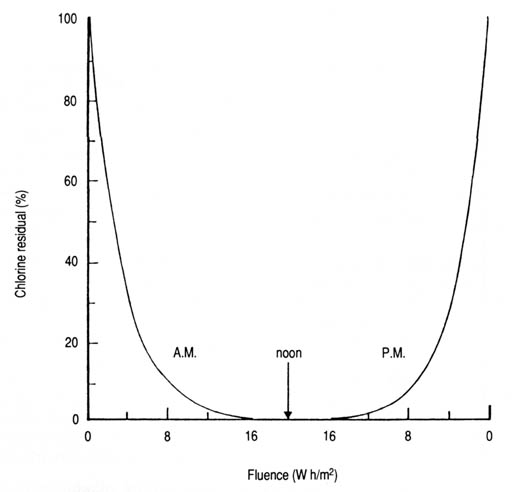 Fig. 31. Reduction in total chlorine residual as a function of solar UV-A fluence throughout the day.
Conclusions and recommendationsThe developed halosol process was tested on a pilot scale and has proven to be a functional and simple technique for water disinfection. With some refinements, field testing, and assessment of costs and acceptability, its application in developing countries with adequate sunlight and a high risk of waterborne diseases could become a reality. By replicating the solar reactor modules, it would be feasible to increase the capacity and productivity of the units. Installing a radiometric device to synchronize the water flow with the variable solar intensity is feasible and desirable.Some areas needing research that emerged during this study are the following:
|